What is Empirical Formula
Multiply each elements atomic mass by the number of atoms of that element in the molecule. Therefore in chemistry the elements and compounds are represented in abbreviated forms.

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
The complicated formula above breaks down in the following way.

. Quantifying the evidence or making sense of it in. A simple example of this concept is that the empirical formula of sulfur monoxide or SO would simply be SO as is the empirical formula of disulfur dioxide S 2 O 2Thus sulfur monoxide and disulfur dioxide both compounds of sulfur and oxygen have the. The molar mass of nicotine is 1621 gmol.
How to calculate the Empirical Formula from Element Proportions. Basically the mass of the empirical formula can be computed by dividing the molar mass of the compound by it. A periodic table will be required to complete this test.
This 10-question practice test deals with finding empirical formulas of chemical compounds. Sometimes the empirical formula and molecular formula both can be the same. A chemical formula is a simple representation in writing of a three dimensional molecule that exists.
Empiricism values some research more than other kinds. Use the periodic table to determine the atomic mass of each element in the molecule. A periodic table will be required to complete this practice test.
A compounds empirical formula is the simplest written expression of its elemental composition. The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound. In statistics the range is the difference between the highest and lowest data value in the set.
The Zipfian distribution is one of a family of related discrete power law probability distributionsIt is related to the zeta distribution but is. We can determine the empirical formula by using the proportion of each element in the compound data. This is used to find one of the measures when the other two measures are known to us for certain data.
This combustion analysis calculator considers the symbol percentage mass of the element determine the simplest whole-number ratio of atoms in. The empirical formula of any compound is the simplest whole-number ratio of each individual type of atom in that compound. Determine the molecular formula of the molecule.
The semi-empirical mass formula states the binding energy is. Still there is another way of representing compounds. The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound.
Find the empirical formula for this compound knowing that H 1 gmole O 16 gmole and C 12 gmole. In statistics the 6895997 rule also known as the empirical rule is a shorthand used to remember the percentage of values that lie within an interval estimate in a normal distribution. Empirical evidence the record of ones direct observations or experiences can be analyzed quantitatively or qualitatively.
A compound is composed of 40 carbon 667 hydrogen and 533 oxygen. In chemistry the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. The molecular formula is the representation of the actual whole number ratio between the elements of the compound.
A compound is composed of 40 carbon 667 hydrogen and 533 oxygen. Is an empirical law formulated using mathematical statistics that refers to the fact that for many types of data studied in the physical and social sciences the rank-frequency distribution is an inverse relation. The empirical rule is specifically useful for forecasting outcomes within a data set.
The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound but not the actual numbers of atoms found in the molecule. Empirical research is research using empirical evidenceIt is also a way of gaining knowledge by means of direct and indirect observation or experience. 68 95 and 997 of the values lie within one two and three standard deviations of the mean respectively.
This step-by-step tutorial shows how to calculate the empirical and molecular formulas for a. To do this all you have to do is write the letters of each component in this case C for carbon H for hydrogen and O for oxygen with their whole number counter parts as subscripts. In the study of a chemical system we need to represent elements and compounds very frequently.
The term is either zero or depending on the parity of and where for some exponent. You should be able to determine the empirical formula for any compound as long as you know the mass of each element present the percentage of mass for each present element or the molecular formula of the compound. First the standard deviation must be calculated.
The empirical formula for our example is. This number is represented by the subscript next to the element symbol in the molecular formula. The empirical formula of a hydrocarbon compound that contains only C and H is found to be CH.
We can determine the empirical formula by using the proportion of each element in the compound data. The ratios are denoted by subscripts next to the element symbols. C 3 H 4 O 3.
Answers for the test appear after the final question. We will talk about what empirical formula and molecular formula are how they are different and well learn how to write the empirical formula for a compoun. This relationship is rewritten in different forms by interchanging the LHS and RHS.
Determine the mean of the data set which is the total of the data set divided by the quantity of numbers. The mass of an atomic nucleus for neutrons protons and therefore nucleons is given by where and are the rest mass of a proton and a neutron respectively and is the binding energy of the nucleus. Multiply every atom subscripts by this ratio to compute the molecular formula.
How to calculate the Empirical Formula from Element Proportions. This 10-question practice test deals with finding the molecular formula of chemical compounds. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines.
Find the empirical formula for this compound knowing that H 1 gmole O 16 gmole and C 12 gmole. A chemical formula describes a substance down to the exact atoms which make it up. The abbreviated representation of an element or a compound is called chemical formula.
Zipfs law z ɪ f German. An online empirical formula calculator allows you to find empirical formula corresponding to the given chemical composition. There are three basic types of chemical formula the empirical formula the molecular formula and the structural formula.
We can derive a general expression as Molecular formula n empirical formula where n is a whole number. To find the ratio between the molecular formula and the empirical formula. The empirical formula of Boron.
Empirical Formula Molecular Formula of Butane Octanecaption C 6 H 12 O 6 6 CH 2 O. We may calculate the empirical formula from information about the mass of each element in that. Concept of Empirical Formula.
It contains 740 carbon 87. Answers appear after the final question. This relation is also called an empirical relationship.
Laboratory procedures have found that the molar mass of the compound is 78 gmol. The formula is given below. This lets us find the most appropriate writer for any type of assignment.
What is the molecular formula of this compound. The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound. But it is not possible always.
It may be the same as the compounds molecular formula sometimes. Write down the empirical formula. In mathematical notation these facts can be expressed as follows where Pr is.
A compound contains 8879 oxygen O and 1119.

Calculating Empirical Formula Mcat Study Chemistry Labs Calculus

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Calculating Empirical Formula Chemistry Math Formula
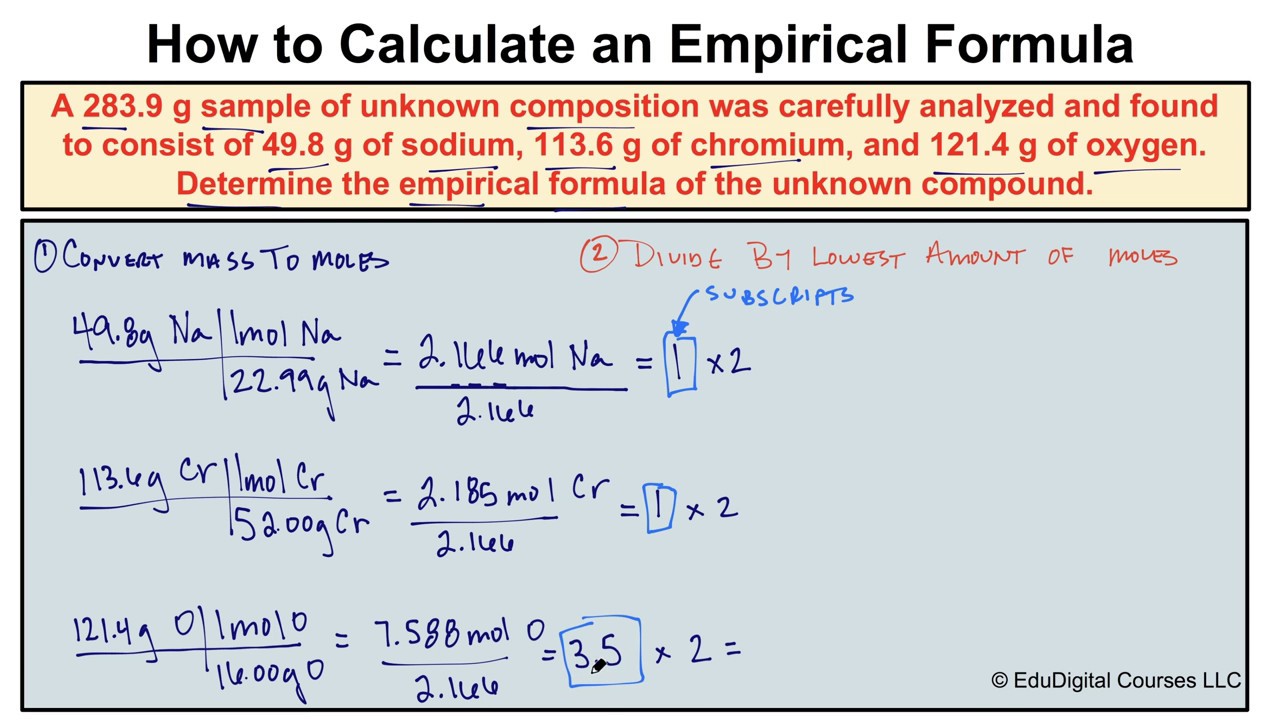
How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Scientific Method Worksheet
No comments for "What is Empirical Formula"
Post a Comment